Pre Forum Courses: 19 October 2022
From a successful prior elicitation meeting to a reliable probability of success estimate: QDM principles and practicalities
Marco Costantini, Luca Grassano, Giulia Zigon (GSK Vaccines)
Pharmaceutical companies too frequently base their investment decisions on single study outcomes, often observed in over optimistic early-stage phases of the clinical development. Such a common bad practice is one of the leading factors to failing in pivotal confirmatory studies. Implementation of QDM makes it possible to incorporate existing estimates of uncertainty into a sound and quantitative evaluation of the risk of success/unsuccess for ongoing and future clinical trials.
This course aims at describing the key elements of Quantitative Decision-Making (QDM), by introducing the theoretical framework and providing practical examples through case-studies. At the end of the course, it will be clear how QDM, where fully acknowledged and formally inserted in the company governance, can increase the chances of obtaining marketing authorization for drugs and therapies under development while reducing wrong decisions of investment on candidates with low probability of success
Validation in Statistical Programming: Way of Working QC and Applied Exercises
Gabriele Di Domenico, Stefano Lombardi, Valerio Romolini(GSK Vaccines)
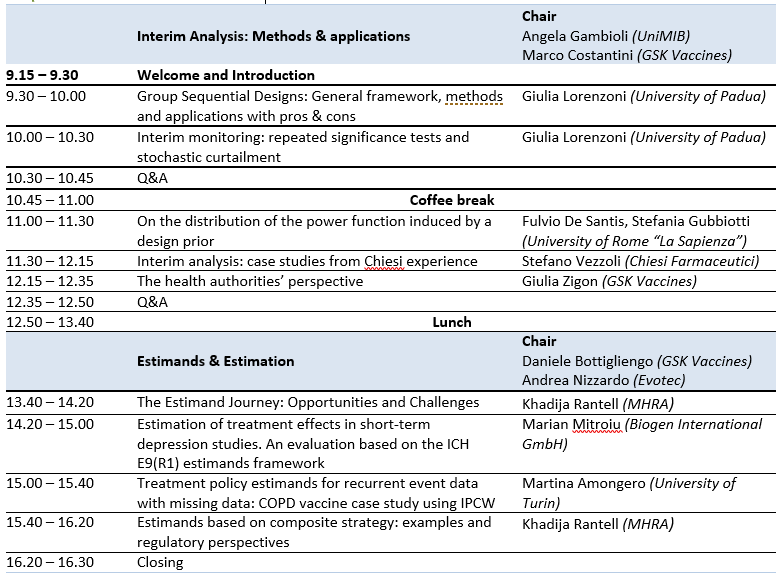
Day 1: Statistics Beyond Clinical Trials
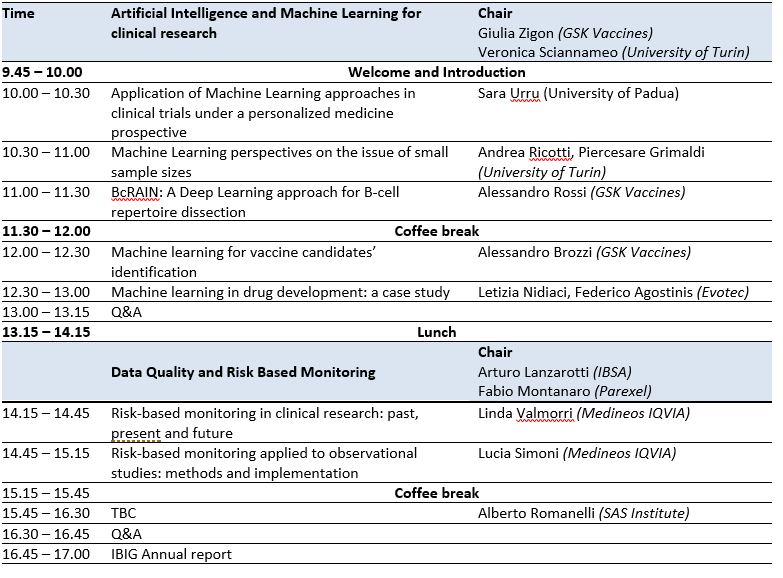
Day 2: Statistics in Clinical Trials